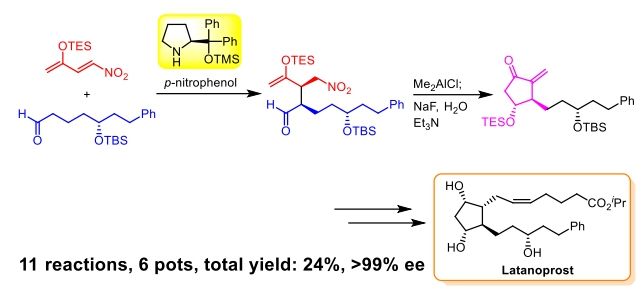
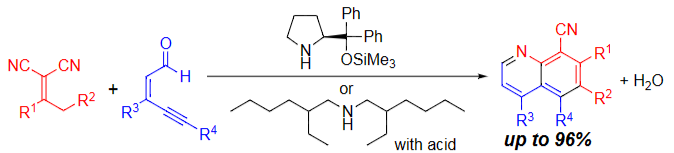
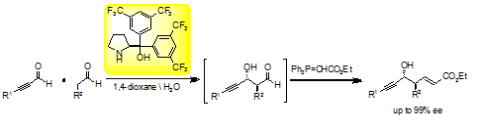
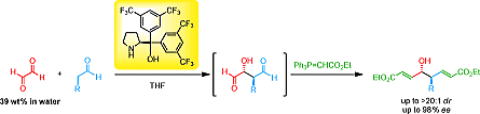
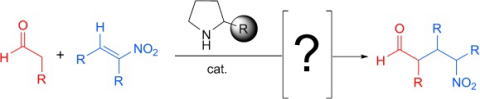
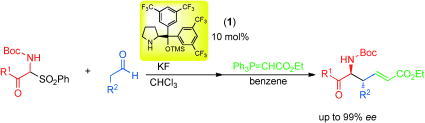
Publication list was updated.
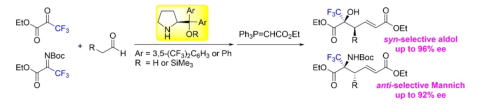
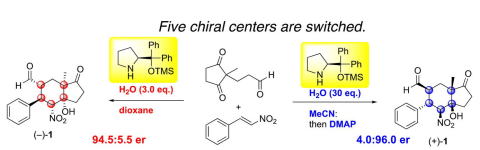
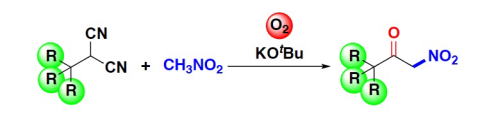
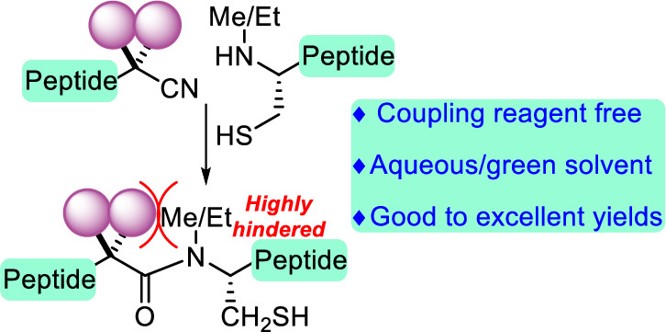
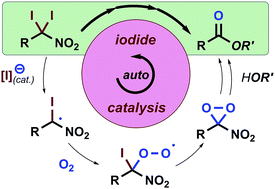
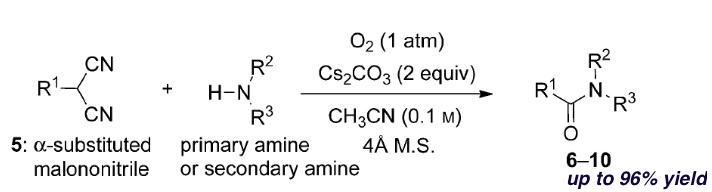
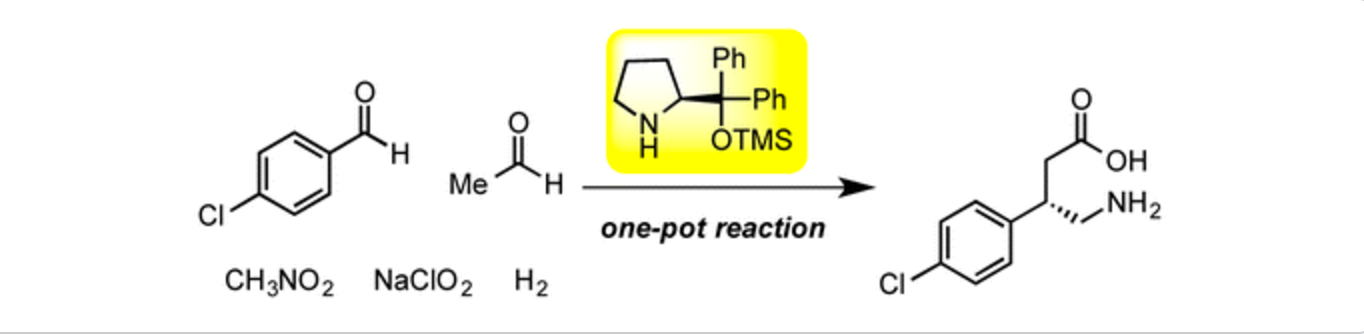
Oxidative peptide bond formation of glycine–amino acid
using 2-(aminomethyl)malononitrile as a glycine unit
Xiaoling Wang, Jing Li and Yujiro Hayashi*
Chem. Commun., 2021, 57, 4283.
DOI: 10.1039/D1CC00130B
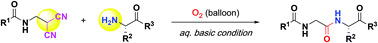
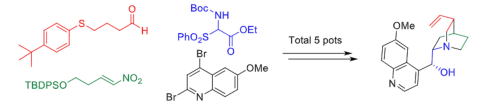
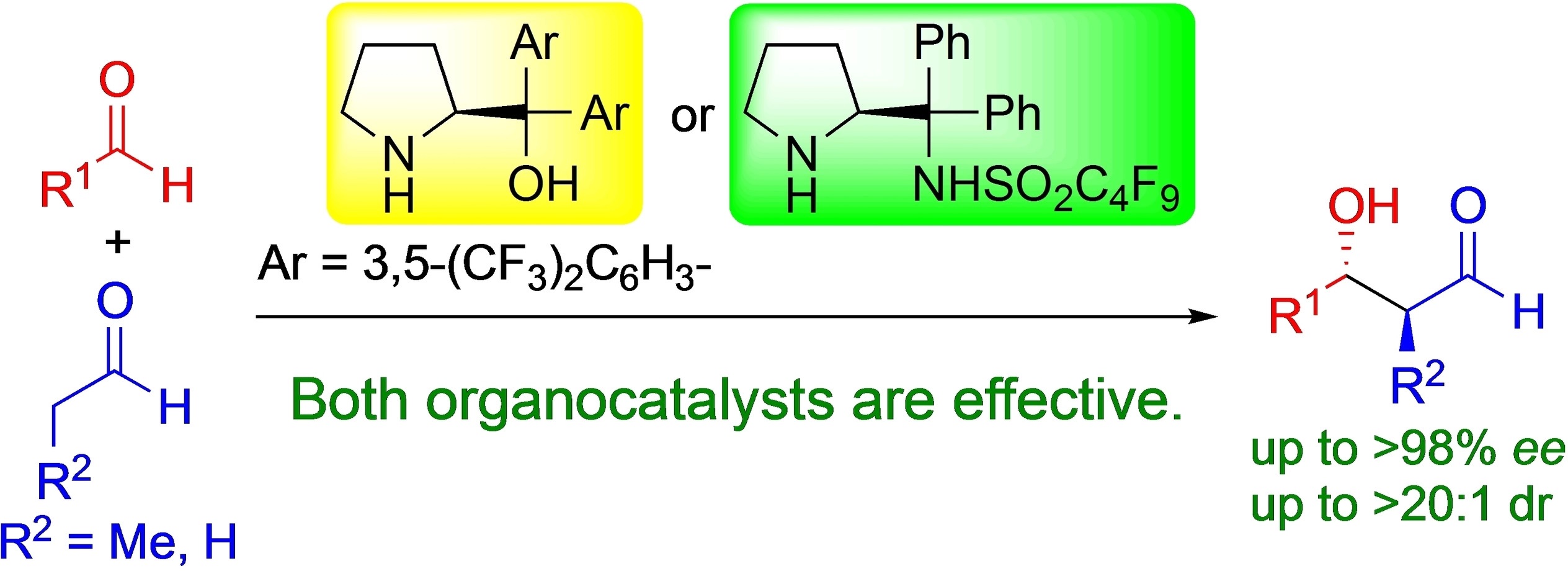
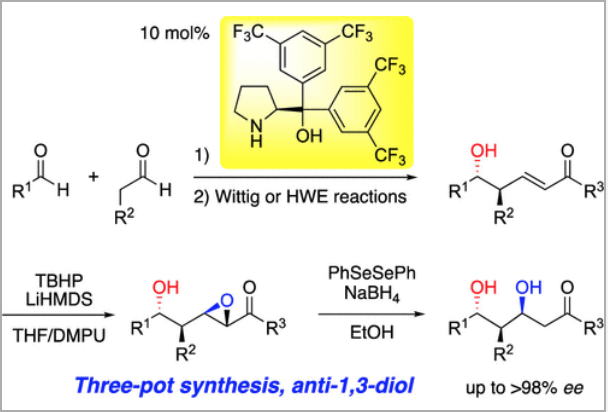
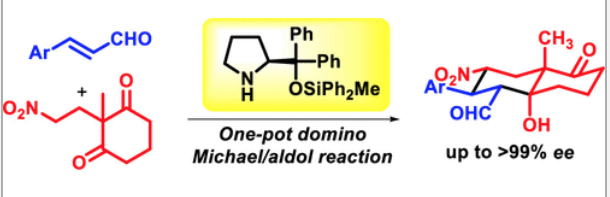
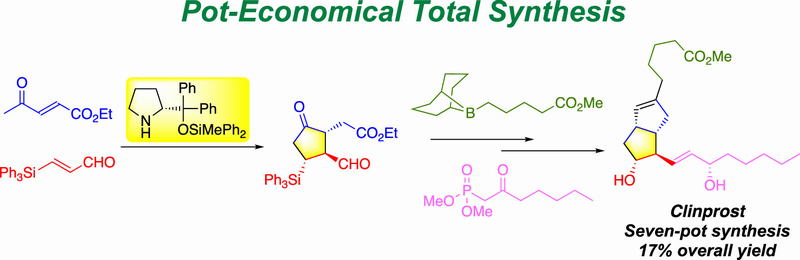
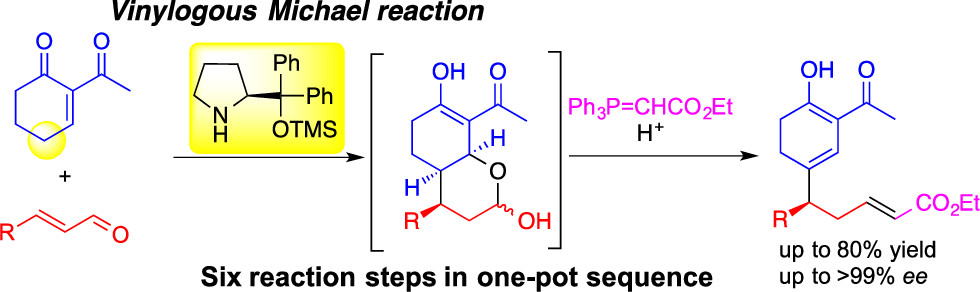
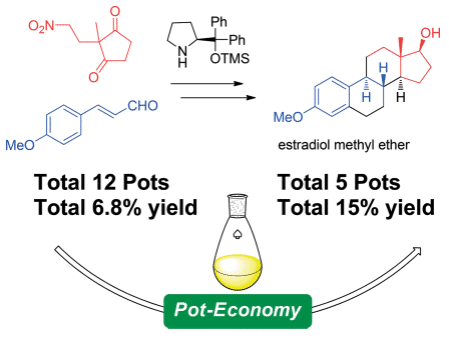
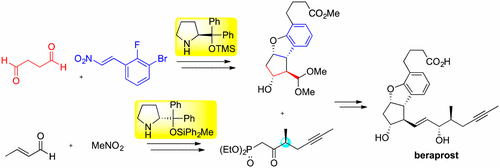
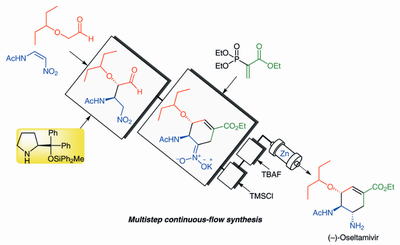
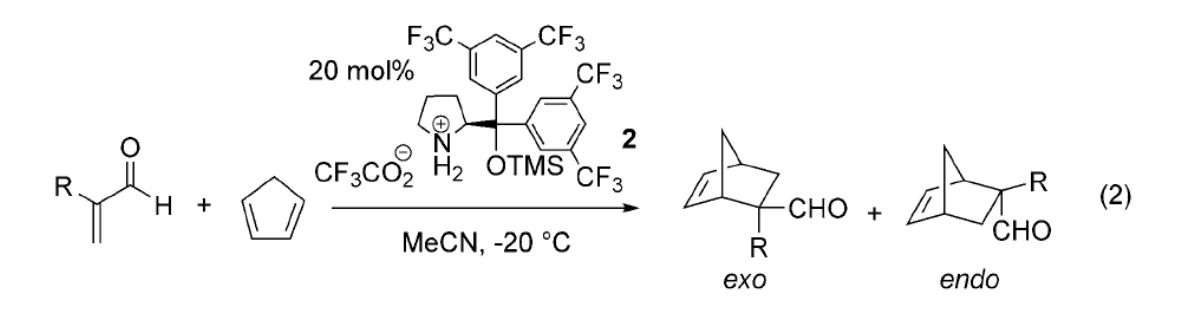
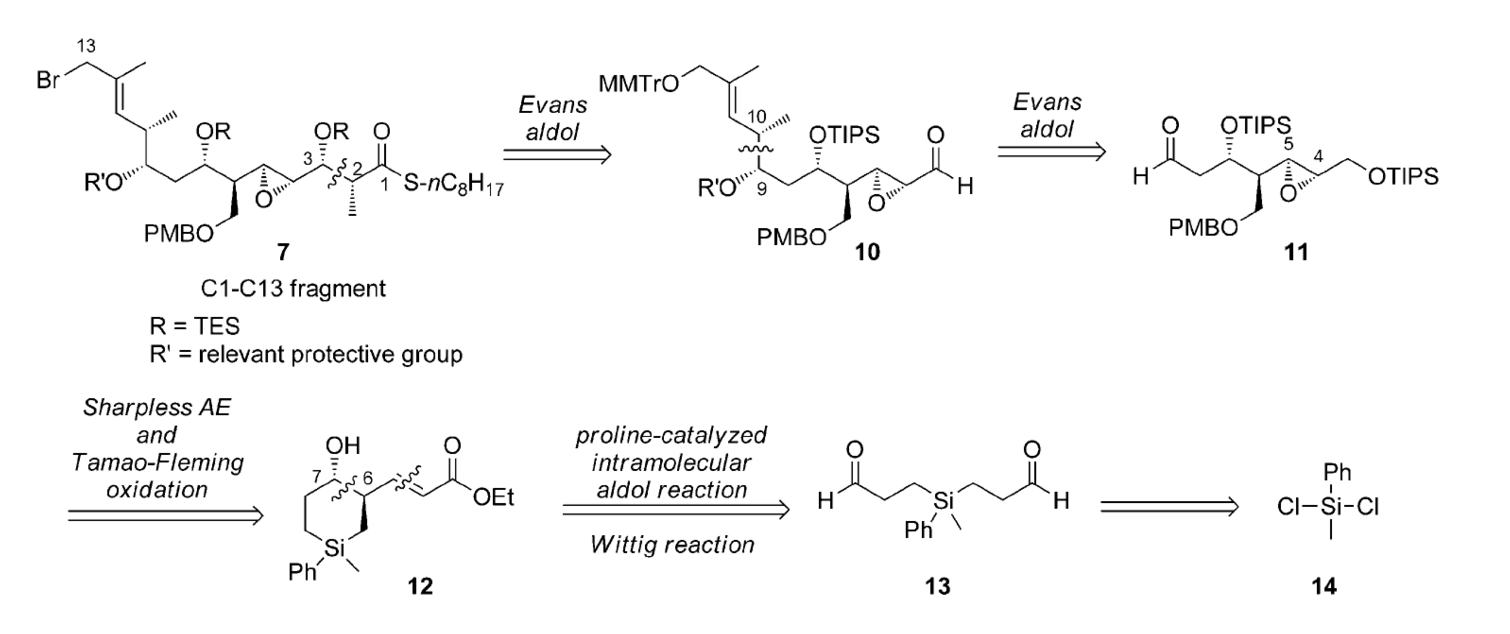
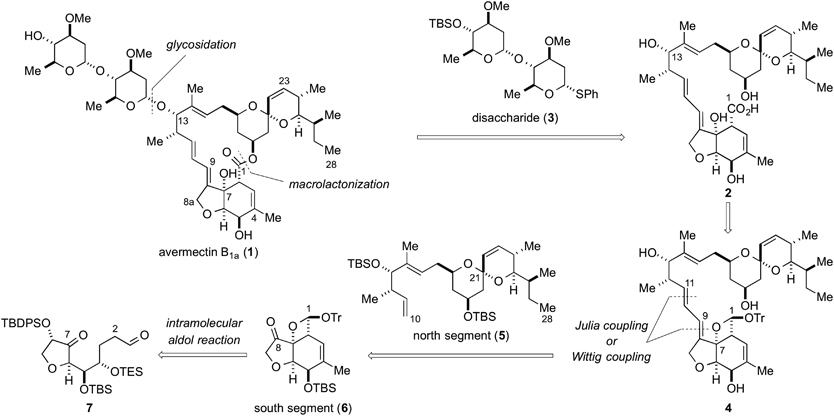
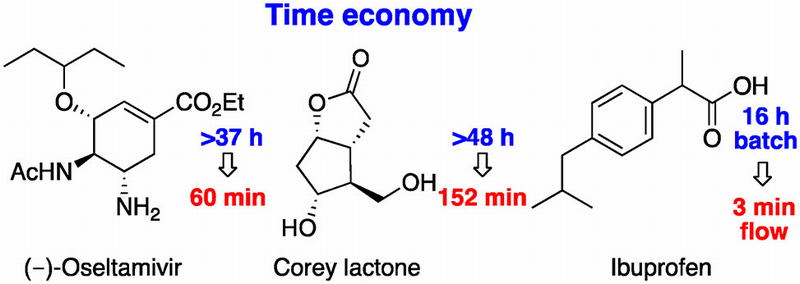
Time Economy in Total Synthesis
Yujiro Hayashi*
J. Org. Chem. 2021, 86, 1.
This article was selected as cover picture.
https://dx.doi.org/10.1021/acs.joc.0c01581

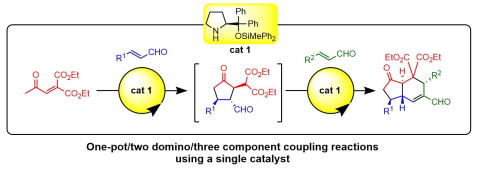
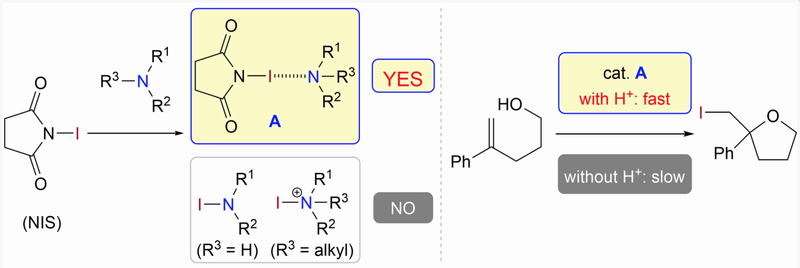
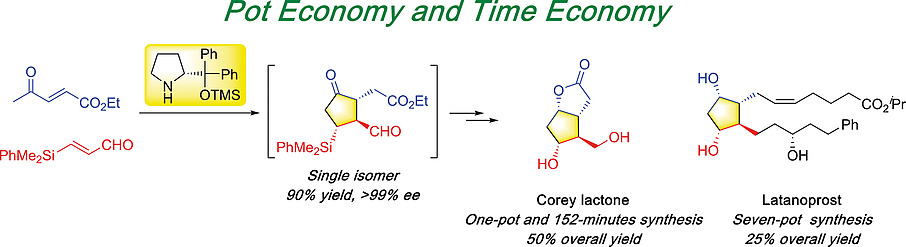
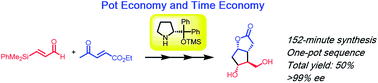
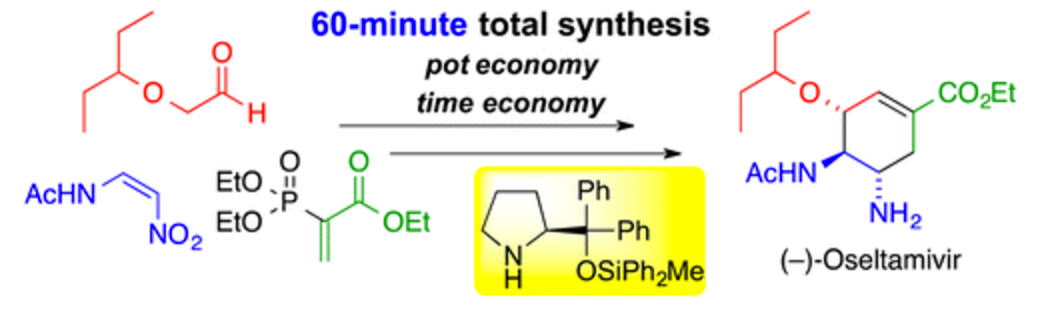
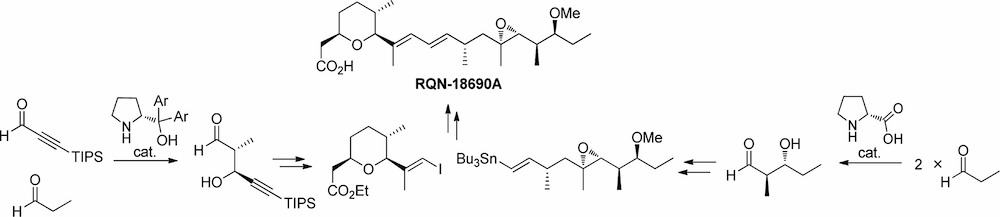
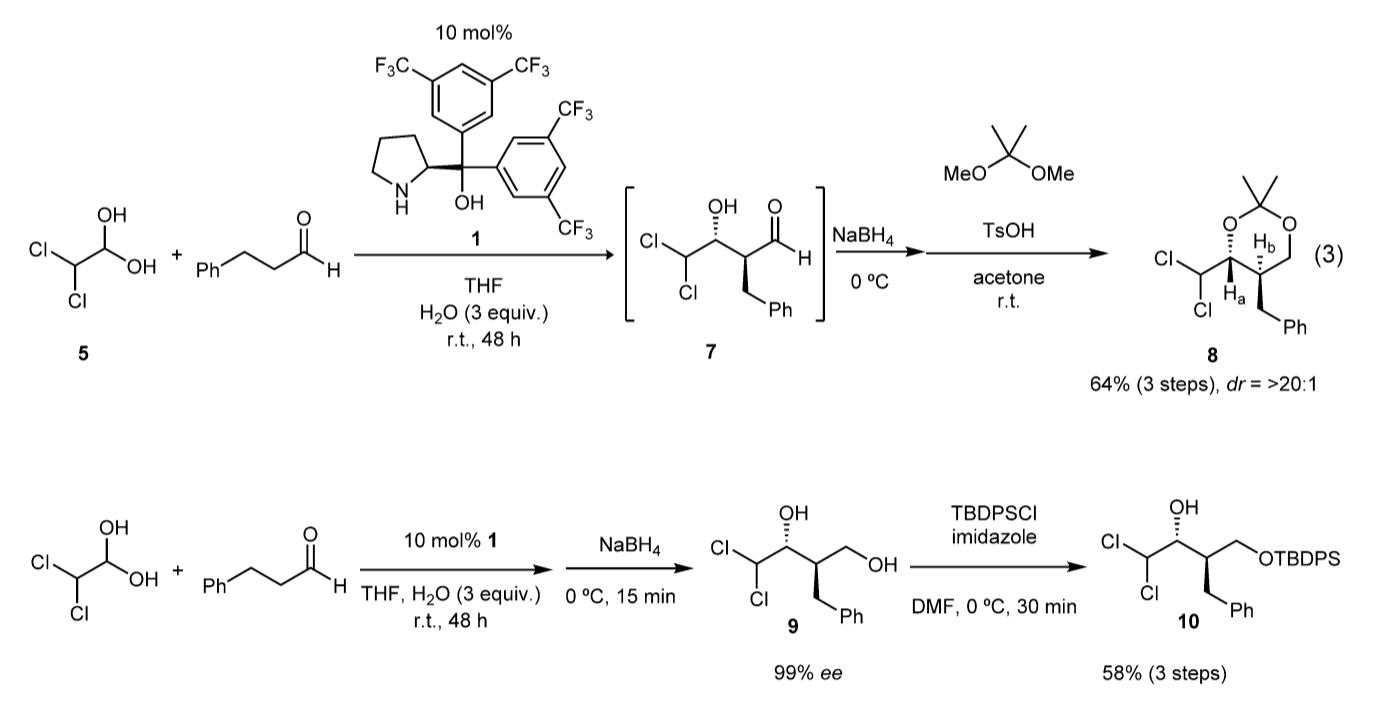
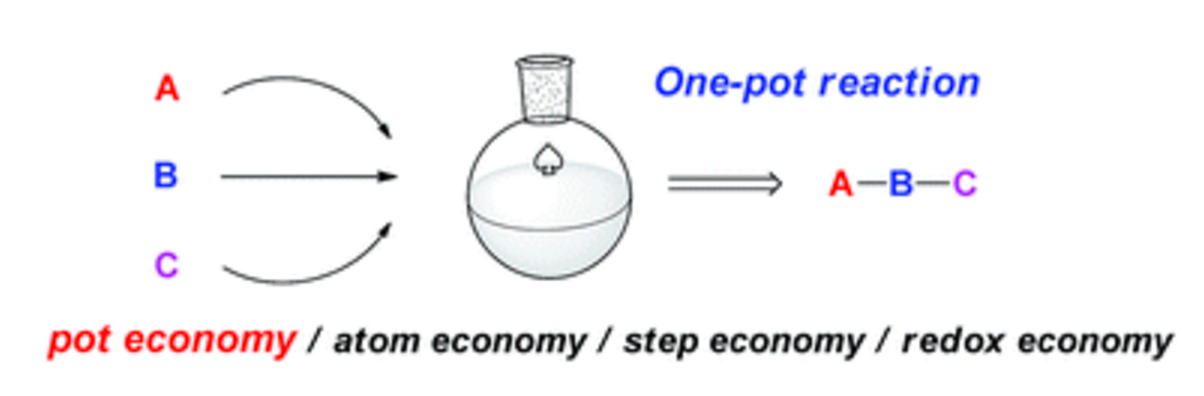
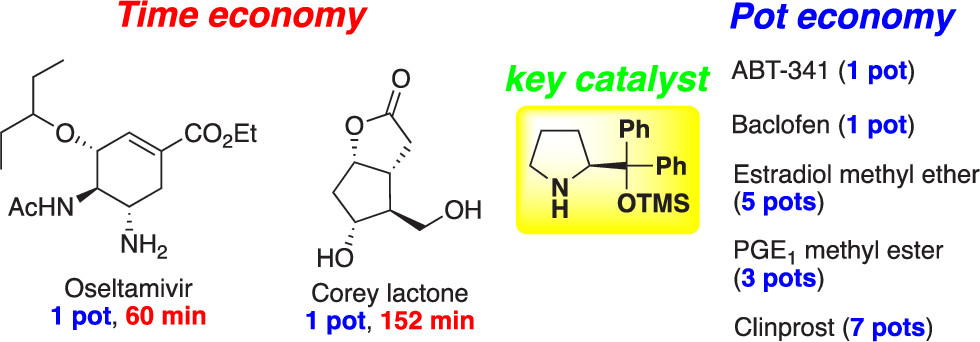
Time and Pot Economy in Total Synthesis
Yujiro Hayashi
Acc. Chem. Res. 2021, 54, 1385.
https://doi.org/10.1021/acs.accounts.0c00803
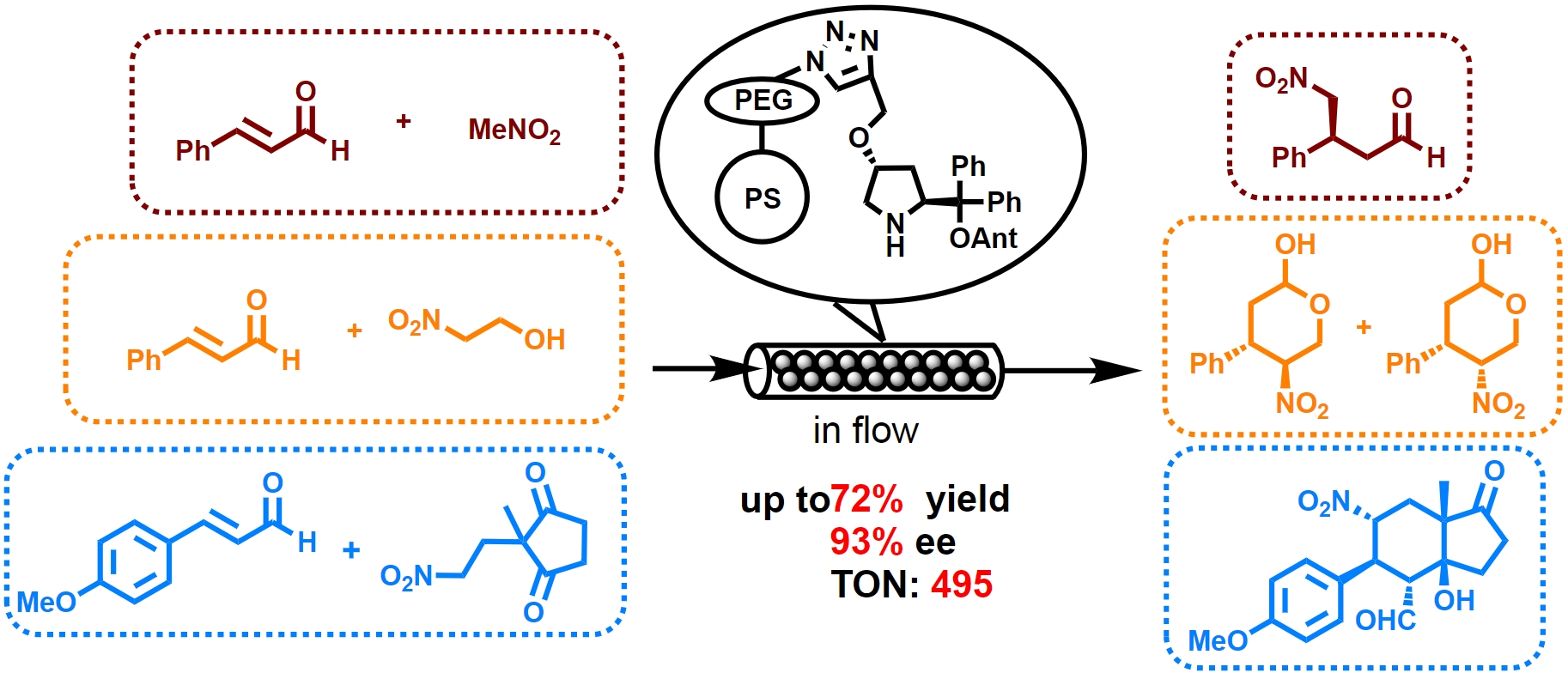
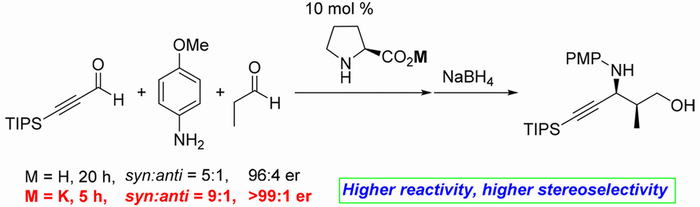
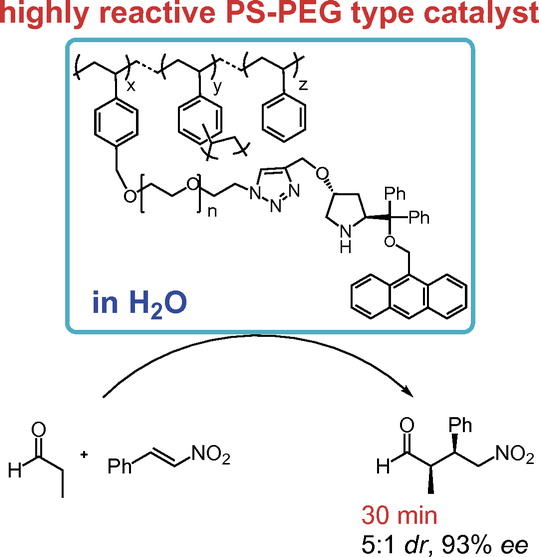
Amphiphilic Immobilized Diphenylprolinol Alkyl Ether Catalyst on PS-PEG Resin
Seitaro Koshino, Shusuke Hattori, Shota Hasegawa, Naoki Haraguchi, Takeshi
Yamamoto,
Michinori Suginome, Yasuhiro Uozumi, and Yujiro Hayashi *
Bull. Chem. Soc. Jpn. 2021, 94, 790.
https://doi.org/10.1246/bcsj.20200355
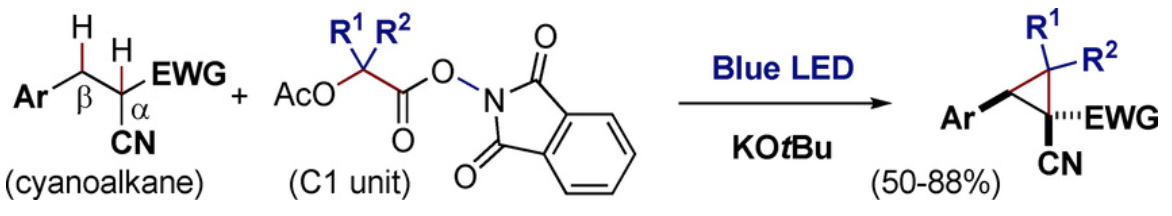
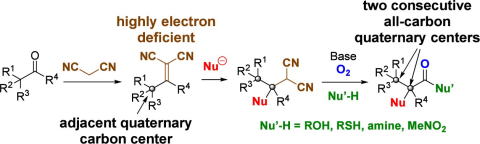
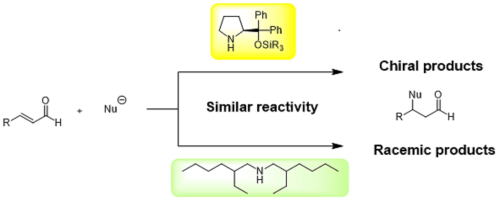
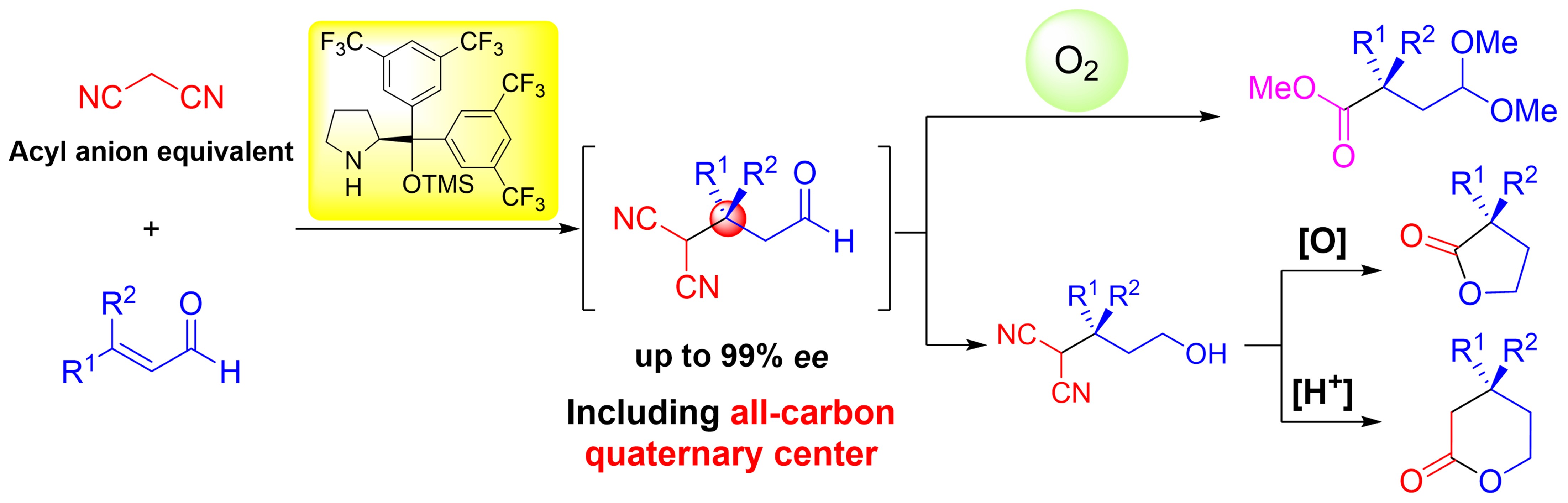
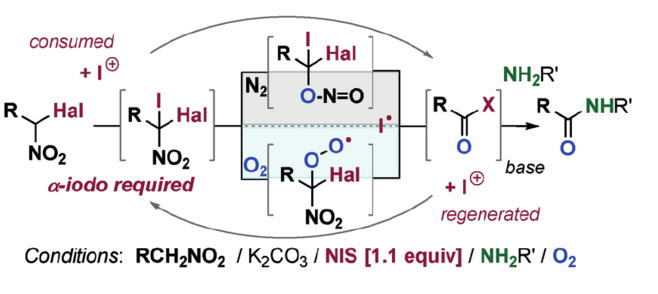
Direct Cyclopropanation of α‐Cyano β‐Aryl Alkanes by Light‐Mediated
Single Electron Transfer Between Donor–Acceptor Pairs
Jing Li, Martin J. Lear, Yujiro Hayashi*
Chem. Eur. J. 2021, 27, 5901.
https://doi.org/10.1002/chem.202100341